Aromatization modulates the activity of small organic molecules as promoters for carbon–halogen bond activation†
Abstract
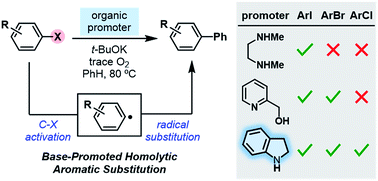
The combination of small organic molecules and a base serves as a unique system for the activation carbon–halogen bonds in haloarenes by single electron transfer (SET). However, most of the molecules employed as promoters only allow for the activation of aryl iodides, and efficient activation of aryl bromides and chlorides under this mode is still rather challenging. Herein, we report the discovery of a structurally simple yet powerful promoter molecule, indoline, which exhibits unusually high activity in promoting the activation of haloarenes by SET. In the presence of t-BuOK and a trace amount of oxygen, indoline promotes the formation of aryl radicals not only from aryl iodides and bromides, but also from unactivated aryl chlorides (e.g., chlorobenzene) under relatively mild conditions. Mechanistic studies reveal the molecular basis for its high activity, for which the aromatization process plays a key role in modulating the electron transfer process.


 Please wait while we load your content...
Please wait while we load your content...