Absolute and relative facial selectivities in organocatalytic asymmetric chlorocyclization reactions†
Abstract
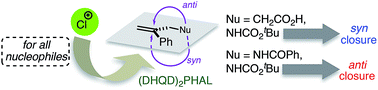
Though (DHQD)2PHAL-catalyzed chlorocyclizations of 1,1-disubstituted olefins show useful (and in some cases, reversible) asymmetric induction, stereochemically complete descriptions of these alkene additions have remained largely unknown. Herein, based on a combination of NMR, derivative, isotope labeling, and computational studies, we present detailed stereochemical analyses of chlorocyclizations of nucleophile-tethered 1,1-disubstituted styryl systems. The selectivities of the two asymmetric bond-forming processes, namely electrophilic chlorine attack and nucleophilic ring closure, are thus mapped out independently. Under the established optimal conditions, four related chlorocyclizations were subjected to this analysis. All showed a strong preference for Cl+ delivery from the same face of the alkene. However, depending on reaction conditions and substrate identity (carboxylic acid, amide or carbamate), the internal nucleophiles may close with a strong net preference for either syn or anti addition relative to the Cl atom. Studies of both uncatalyzed and (DHQD)2PHAL-catalyzed processes place new boundary conditions on the role of the catalyst in these reactions.


 Please wait while we load your content...
Please wait while we load your content...