Exploring the role of ionic liquids to tune the polymorphic outcome of organic compounds†
Abstract
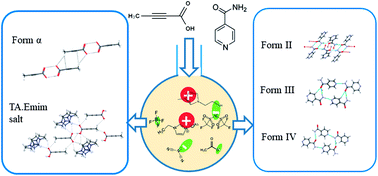
While molecular solvents are commonly used in the screening of polymorphs, the choices are often restricted. Ionic liquids (ILs) – also referred as designer solvents – have immense possibility in this regard because of their wide flexibility of tunability. More importantly, the interactions among the IL components are completely unique compared to those present in the molecular solvents. In this context, we have chosen tetrolic acid (TA) and isonicotinamide (INA), which showed solution-structure link in molecular solvents in the past, as probes to investigate the role of imidazolium based ionic liquids in the polymorphism of these two systems and whether the different solute–solvent interactions in ILs affect the polymorphic outcome. It is observed that the selected imidazolium-based ILs, with varying anion basicity have influenced the crystallization outcome by the interaction between ILs and model compounds. Later, we have utilized the concept of double salt ionic liquids (DSIL) for INA, a penta-morphic system, to investigate the variation in the polymorphic outcome. This approach helped to obtain the forms that were otherwise inaccessible in ILs.


 Please wait while we load your content...
Please wait while we load your content...