Scalable thioarylation of unprotected peptides and biomolecules under Ni/photoredox catalysis†
Abstract
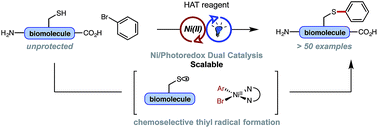
Site-specific functionalization of unprotected native peptides and biomolecules remains a useful transformation in synthetic design and chemical biology, yet until recently, advancements in transition metal-catalyzed methods, which have prevailed in organic synthesis, have been relatively ineffective when applied to large and structurally complex biomolecules. Here, the mechanistically distinct, Ni/photoredox-catalyzed arylation of unprotected, native thiols (e.g., cysteine residues) is reported – a process initiated through a visible light-promoted, hydrogen atom transfer (HAT) event under ambient conditions. Sub-stoichiometric loadings of the dual-catalyst system (≤5 mol%) are employed, granting excellent site-specificity, broad substrate scope, and low chemical waste. Reaction scalability (from μg to grams) has been achieved through modest reagent adjustments, and high throughput experimentation (HTE) demonstrates the ease of reaction setup, enabling prompt screening of aryl halide coupling partners and conditions. Scores of thiol substrates and aryl entities were examined and effectively conjugated, suggesting further diverse, practical applications.
- This article is part of the themed collections: Most popular 2018-2019 catalysis articles and 2018 International Open Access Week Collection


 Please wait while we load your content...
Please wait while we load your content...