Proteome-wide mapping of PQS-interacting proteins in Pseudomonas aeruginosa†
Abstract
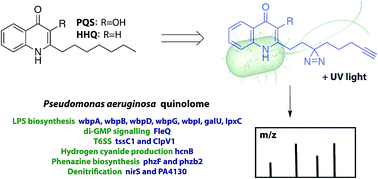
The opportunistic human pathogen Pseudomonas aeruginosa secretes 2-heptyl-3-hydroxy-4-quinolone (PQS), a quorum sensing (QS) signal that regulates the expression of numerous virulence genes. Here we report the development and application of chemical probes to globally map quinolone binding proteins. The revealed quinolone interactome contains both known as well as newly identified virulence factors and presents new targets for the treatment of bacterial infections.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...