Overcoming double-step CO2 adsorption and minimizing water co-adsorption in bulky diamine-appended variants of Mg2(dobpdc)†
Abstract
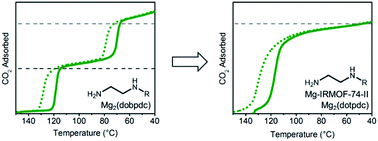
Alkyldiamine-functionalized variants of the metal–organic framework Mg2(dobpdc) (dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) are promising for CO2 capture applications owing to their unique step-shaped CO2 adsorption profiles resulting from the cooperative formation of ammonium carbamate chains. Primary,secondary (1°,2°) alkylethylenediamine-appended variants are of particular interest because of their low CO2 step pressures (≤1 mbar at 40 °C), minimal adsorption/desorption hysteresis, and high thermal stability. Herein, we demonstrate that further increasing the size of the alkyl group on the secondary amine affords enhanced stability against diamine volatilization, but also leads to surprising two-step CO2 adsorption/desorption profiles. This two-step behavior likely results from steric interactions between ammonium carbamate chains induced by the asymmetrical hexagonal pores of Mg2(dobpdc) and leads to decreased CO2 working capacities and increased water co-adsorption under humid conditions. To minimize these unfavorable steric interactions, we targeted diamine-appended variants of the isoreticularly expanded framework Mg2(dotpdc) (dotpdc4− = 4,4′′-dioxido-[1,1′:4′,1′′-terphenyl]-3,3′′-dicarboxylate), reported here for the first time, and the previously reported isomeric framework Mg-IRMOF-74-II or Mg2(pc-dobpdc) (pc-dobpdc4− = 3,3′-dioxidobiphenyl-4,4′-dicarboxylate, pc = para-carboxylate), which, in contrast to Mg2(dobpdc), possesses uniformally hexagonal pores. By minimizing the steric interactions between ammonium carbamate chains, these frameworks enable a single CO2 adsorption/desorption step in all cases, as well as decreased water co-adsorption and increased stability to diamine loss. Functionalization of Mg2(pc-dobpdc) with large diamines such as N-(n-heptyl)ethylenediamine results in optimal adsorption behavior, highlighting the advantage of tuning both the pore shape and the diamine size for the development of new adsorbents for carbon capture applications.
- This article is part of the themed collections: How can chemistry adapt to a low carbon future, Editor’s Choice – Jihong Yu and International Symposium on Macrocyclic & Supramolecular Chemistry (ISMSC) in conjunction with ISACS


 Please wait while we load your content...
Please wait while we load your content...