Models for aerobic carbon monoxide dehydrogenase: synthesis, characterization and reactivity of paramagnetic MoVO(μ-S)CuI complexes†
Abstract
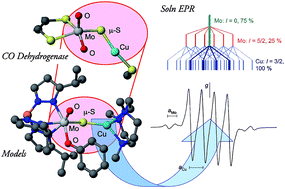
Reaction of [CoCp2][TpiPrMoOS(OAr)] [Cp = η5-cyclopentadienyl; TpiPr = hydrotris(3-isopropylpyrazol-1-yl)borate; OAr = phenolate or derivative thereof] with [Cu(NCMe)(Me3tcn)]BF4 (Me3tcn = 1,4,7-trimethyl-1,4,7-triazacyclononane) in MeCN at −30 °C results in the formation of red-brown/black, paramagnetic, μ-sulfido-Mo(V)/Cu(I) complexes, TpiPrMoO(OAr)(μ-S)Cu(Me3tcn). The complexes possess the MoO(μ-S)Cu core found in aerobic carbon monoxide dehydrogenases (CODHs) and exhibit X-band EPR spectra closely related to those of semi-reduced CODH, with giso ∼ 1.937, hyperfine coupling to 95,97Mo (aiso = 39–42 × 10−4 cm−1) and strong superhyperfine coupling to 63,65Cu (aiso = 34–63 × 10−4 cm−1). Anisotropic spectra exhibit monoclinic symmetry with g1 ∼ 1.996, g2 ∼ 1.944 and g3 ∼ 1.882, and nearly isotropic ACu values (75–90 × 10−4 cm−1). The X-ray structures of four derivatives (Ar = Ph, C6H4tBu-2, C6H4sBu-2, C6H4Ph-4) are reported and discussed along with that of the Ar = C6H3tBu2-3,5 derivative (communicated in C. Gourlay, D. J. Nielsen, J. M. White, S. Z. Knottenbelt, M. L. Kirk and C. G. Young, J. Am. Chem. Soc., 2006, 128, 2164). The complexes exhibit distorted octahedral oxo-Mo(V) and distorted tetrahedral Cu(I) centres bridged by a single bent μ-sulfido ligand, with Mo–S and Cu–S distances and Mo–S–Cu angles in the ranges 2.262–2.300 Å, 2.111–2.134 Å and 115.87–134.27°, respectively. The 2 t-butyl derivative adopts a unique phenolate conformation with O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mo–O–Cα and O
Mo–O–Cα and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mo–S–Cu torsion angles of 92.7 and 21.1°, respectively, very different from those of the other structurally characterized derivatives (31–47 and 33–45°, respectively) and exhibits a relatively short Mo⋯Cu distance [3.752(2) Å vs. 3.806(7)–4.040(2) Å]. As well, the aCu value of this complex (34.3 × 10−4 cm−1) is much lower than the values observed for other members of the series (55–63 × 10−4 cm−1), supporting the hypothesis that the electronic structure of the MoO(μ-S)Cu core unit and the degree of intermetallic communication are strongly dependent on the geometry of the MoO(OR)(μ-S)Cu unit. The complexes participate in an electrochemically reversible Mo(VI)/Mo(V) redox couple and react with cyanide undergoing decupration and desulfurization reactions of the type observed for CODH.
Mo–S–Cu torsion angles of 92.7 and 21.1°, respectively, very different from those of the other structurally characterized derivatives (31–47 and 33–45°, respectively) and exhibits a relatively short Mo⋯Cu distance [3.752(2) Å vs. 3.806(7)–4.040(2) Å]. As well, the aCu value of this complex (34.3 × 10−4 cm−1) is much lower than the values observed for other members of the series (55–63 × 10−4 cm−1), supporting the hypothesis that the electronic structure of the MoO(μ-S)Cu core unit and the degree of intermetallic communication are strongly dependent on the geometry of the MoO(OR)(μ-S)Cu unit. The complexes participate in an electrochemically reversible Mo(VI)/Mo(V) redox couple and react with cyanide undergoing decupration and desulfurization reactions of the type observed for CODH.


 Please wait while we load your content...
Please wait while we load your content...