A general method for the metal-free, regioselective, remote C–H halogenation of 8-substituted quinolines†
Abstract
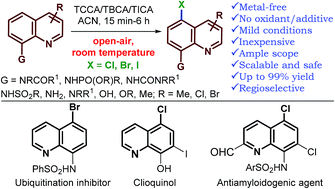
An operationally simple and metal-free protocol for geometrically inaccessible C5–H halogenation of a range of 8-substituted quinoline derivatives has been established. The reaction proceeds under air, with inexpensive and atom economical trihaloisocyanuric acid as a halogen source (only 0.36 equiv.), at room temperature. Exceptionally high generality with respect to quinoline is observed, and in most instances, the reaction proceeded with complete regioselectivity. Quinoline with a variety of substituents at the 8-position gave, exclusively, the C5-halogenated product in good to excellent yields. Phosphoramidates, tertiary amides, N-alkyl/N,N-dialkyl, and urea derivatives of quinolin-8-amine as well as alkoxy quinolines were halogenated at the C5-position via remote functionalization for the first time. This methodology provides a highly economical route to halogenated quinolines with excellent functional group tolerance, thus providing a good complement to existing remote functionalization methods of quinolin-8-amide derivatives and broadening the field of remote functionalization. The utility of the method is further showcased through the synthesis of several compounds of biological and pharmaceutical interest.
- This article is part of the themed collection: Most popular 2018-2019 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...