A catalytic highly enantioselective allene approach to oxazolines†
Abstract
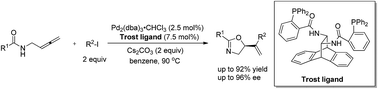
Oxazolines are a very important class of heterocyclic compounds. However, catalytic enantioselective syntheses are very limited. Here, a highly enantioselective palladium-catalyzed coupling-cyclization of readily available N-(buta-2,3-dienyl) amides with aryl or 1-alkenyl iodides has been developed for the asymmetric construction of oxazoline derivatives. Many synthetically useful functional groups are tolerated in this reaction. The absolute configuration of the chiral center in the products has been established by X-ray diffraction study. A model for prediction of the absolute configuration of the chiral center in the products from this cyclic enantioselective nucleophilic allylation has been proposed. The synthetic potentials based on the unique structure of the products formed have also been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...