Palladium-catalyzed regiodivergent hydroaminocarbonylation of alkenes to primary amides with ammonium chloride†
Abstract
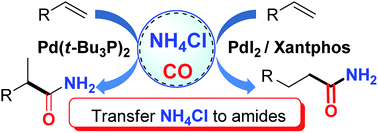
Palladium-catalyzed hydroaminocarbonylation of alkenes for the synthesis of primary amides has long been an elusive aim. Here, we report an efficient catalytic system which enables inexpensive NH4Cl to be utilized as a practical alternative to gaseous ammonia for the palladium-catalyzed alkene-hydroaminocarbonylation reaction. Through appropriate choice of the palladium precursors and ligands, either branched or linear primary amides can be obtained in good yields with good to excellent regioselectivities. Primary mechanistic studies were conducted and disclosed that electrophilic acylpalladium species were capable of capturing the NH2-moiety from ammonium salts to form amides in the presence of CO with NMP as a base.


 Please wait while we load your content...
Please wait while we load your content...