Target-activated streptavidin–biotin controlled binding probe†
Abstract
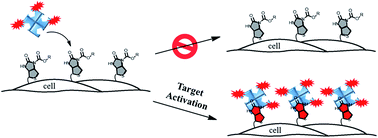
Target-activated chemical probes are important tools in basic biological research and medical diagnosis for monitoring enzyme activities and reactive small molecules. Based on the fluorescence turn-on mechanism, they can be divided into two classes: dye-based fluorescent probes and caged-luciferin. In this paper, we introduce a new type of chemical probe in which the fluorescence turn-on is based on controlled streptavidin–biotin binding. Compared to conventional probes, the streptavidin–biotin controlled binding probe has several advantages, such as minimal background at its “OFF” state, multiple signal amplification steps, and unlimited selection of the optimal dyes for detection. To expand the scope, a new synthetic method was developed, through which a wider range of analyte recognition groups can be easily introduced to construct the binding probe. This probe design was successfully applied to image and study secreted peroxynitrite (ONOO−) at the cell surface of macrophages where information on ONOO− is difficult to obtain. As the signals are generated upon the binding of streptavidin to the biotin probe, this highly versatile design can not only be used in fluorescence detection but can also be applied in various other detection modes, such as electrochemical and enzyme-amplified luminescence detection.


 Please wait while we load your content...
Please wait while we load your content...