Cobalt-catalyzed regioselective stereoconvergent Markovnikov 1,2-hydrosilylation of conjugated dienes†
Abstract
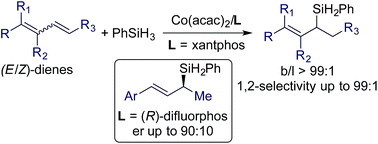
We report the first stereoconvergent Markovnikov 1,2-hydrosilylation of conjugated dienes using catalysts generated from bench-stable Co(acac)2 and phosphine ligands. A wide range of E/Z-dienes underwent this Markovnikov 1,2-hydrosilylation in a stereoconvergent manner, affording (E)-allylsilanes in high isolated yields with high stereoselectivities (E/Z = >99 : 1) and high regioselectivities (b/l up to > 99 : 1). Mechanistic studies revealed that this stereoconvergence stems from a σ–π–σ isomerization of an allylcobalt species generated by the 1,4-hydrometalation of Z-dienes. In addition, a cobalt catalyst that can only catalyze the hydrosilylation of the E-isomer of an (E/Z)-diene was identified, which allows the separation of the (Z)-isomer from an isomeric mixture of (E/Z)-dienes. Furthermore, asymmetric hydrosilylation of (E)-1-aryl-1,3-dienes was studied with Co(acac)2/(R)-difluorphos and good enantioselectivities (er up to 90 : 10) were obtained.
- This article is part of the themed collection: Most popular 2018-2019 catalysis articles


 Please wait while we load your content...
Please wait while we load your content...