Amide synthesis via nickel-catalysed reductive aminocarbonylation of aryl halides with nitroarenes†
Abstract
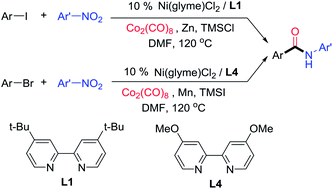
Aminocarbonylation of aryl halides is one of the most useful methods in amide synthesis, but nitroarenes have not been used as a nitrogen source in this method even though they are more economical and accessible than anilines. Reported here is the development of nickel catalysis for the first three-component reactions of aryl halides, Co2(CO)8, and nitroarenes under reductive conditions to produce aryl amides. A wide range of (hetero)aryl iodides and bromides as well as nitro(hetero)arenes are suitable reaction partners, and high functional group compatibility has been achieved. The method might be used for the streamlined synthesis of aryl amides.


 Please wait while we load your content...
Please wait while we load your content...