Guanine–oligothiophene conjugates: liquid-crystalline properties, photoconductivities and ion-responsive emission of their nanoscale assemblies†
Abstract
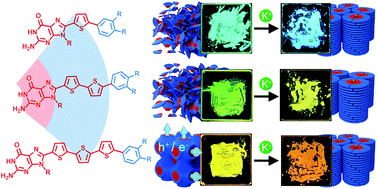
We here report the supramolecular self-assembly of hydrogen-bonded motifs for the development of nanostructured materials that exhibit dynamic functions such as stimuli-responsive properties and molecular recognition behaviour. We have designed and synthesised new thermotropic bicontinuous and columnar liquid-crystalline (LC) guanine–oligothiophene conjugates tethered with lipophilic chains, which exhibit ionic, electronic and photoluminescence properties. Their potassium salt complexes self-assemble into thermotropic columnar LC phases. Time-of-flight photoconductivity measurements have revealed that the guanine–oligothiophene conjugates in the LC states possess charge transport abilities with either electron or ambipolar mobility values of 10−4 to 10−3 cm2 V−1 s−1. Furthermore, we have found that the complexation of potassium ions with the guanine motif could lead not only to structural change and thermal stabilization of the LC phases but also to a photoluminescence colour change in the solid states. The strategy presented in this work could lead to the design of new functional LC materials that could potentially be applicable as sensors and electronic devices.


 Please wait while we load your content...
Please wait while we load your content...