A structurally-characterized peroxomanganese(iv) porphyrin from reversible O2 binding within a metal–organic framework†
Abstract
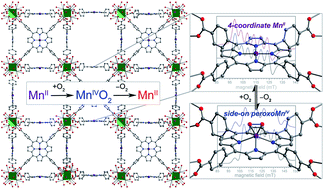
The role of peroxometal species as reactive intermediates in myriad biological processes has motivated the synthesis and study of analogous molecular model complexes. Peroxomanganese(IV) porphyrin complexes are of particular interest, owing to their potential ability to form from reversible O2 binding, yet have been exceedingly difficult to isolate and characterize in molecular form. Alternatively, immobilization of metalloporphyrin sites within a metal–organic framework (MOF) can enable the study of interactions between low-coordinate metal centers and gaseous substrates, without interference from bimolecular reactions and axial ligation by solvent molecules. Here, we employ this approach to isolate the first rigorously four-coordinate manganese(II) porphyrin complex and examine its reactivity with O2 using infrared spectroscopy, single-crystal X-ray diffraction, EPR spectroscopy, and O2 adsorption analysis. X-ray diffraction experiments reveal for the first time a peroxomanganese(IV) porphyrin species, which exhibits a side-on, η2 binding mode. Infrared and EPR spectroscopic data confirm the formulation of a peroxomanganese(IV) electronic structure, and show that O2 binding is reversible at ambient temperature, in contrast to what has been observed in molecular form. Finally, O2 gas adsorption measurements are employed to quantify the enthalpy of O2 binding as hads = −49.6(8) kJ mol−1. This enthalpy is considerably higher than in the corresponding Fe- and Co-based MOFs, and is found to increase with increasing reductive capacity of the MII/III redox couple.


 Please wait while we load your content...
Please wait while we load your content...