Reversible-deactivation anionic alternating ring-opening copolymerization of epoxides and cyclic anhydrides: access to orthogonally functionalizable multiblock aliphatic polyesters†
Abstract
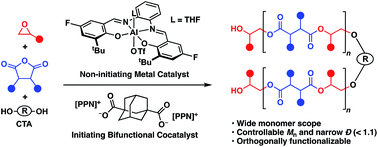
The alternating copolymerization of epoxides and cyclic anhydrides is an increasingly popular route to aliphatic polyesters that are of interest as biodegradable replacements for petroleum-based polymers and for use in the biomedical field. However, broad and bimodal molecular weight distributions in these polymerizations continues to be an issue, limiting synthesis of multiblock copolymers. By use of a bifunctional catalytic system, the reversible-deactivation anionic alternating ring-opening copolymerization of epoxides and cyclic anhydrides gives unimodal polymers with Đ values generally less than 1.07. This allowed for the formation of well-defined triblock copolymers. Additionally, by incorporating both aldehyde and alkene functionalities into the polymer, orthogonal post-polymerization modification was achieved, giving access to well-defined highly modifiable aliphatic polyesters.


 Please wait while we load your content...
Please wait while we load your content...