Influences of the heme-lysine crosslink in cytochrome P460 over redox catalysis and nitric oxide sensitivity†
Abstract
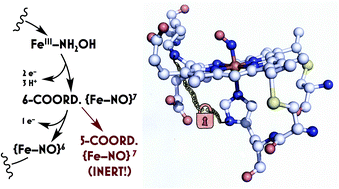
Ammonia (NH3)-oxidizing bacteria (AOB) derive total energy for life from the multi-electron oxidation of NH3 to nitrite (NO2−). One obligate intermediate of this metabolism is hydroxylamine (NH2OH), which can be oxidized to the potent greenhouse agent nitrous oxide (N2O) by the AOB enzyme cytochrome (cyt) P460. We have now spectroscopically characterized a 6-coordinate (6c) {FeNO}7 intermediate on the NH2OH oxidation pathway of cyt P460. This species has two fates: it can either be oxidized to the {FeNO}6 that then undergoes attack by NH2OH to ultimately generate N2O, or it can lose its axial His ligand, thus generating a stable, off-pathway 5-coordinate (5c) {FeNO}7 species. We show that the wild type (WT) cyt P460 exhibits a slow nitric oxide (NO)-independent conversion (kHis-off = 2.90 × 10−3 s−1), whereas a cross-link-deficient Lys70Tyr cyt P460 mutant protein underwent His dissociation via both a NO-independent (kHis-off = 3.8 × 10−4 s−1) and a NO-dependent pathway [kHis-off(NO) = 790 M−1 s−1]. Eyring analyses of the NO-independent pathways for these two proteins revealed a significantly larger (ca. 27 cal mol−1 K−1) activation entropy (ΔS‡) in the cross-link-deficient mutant. Our results suggest that the Lys–heme cross-link confers rigidity to the positioning of the heme P460 cofactor to avoid the fast NO-dependent His dissociation pathway and subsequent formation of the off-pathway 5c {FeNO}7 species. The relevance of these findings to NO signaling proteins such as heme-nitric oxide/oxygen binding (H-NOX) is also discussed.


 Please wait while we load your content...
Please wait while we load your content...