Deciphering the incognito role of water in a light driven proton coupled electron transfer process†
Abstract
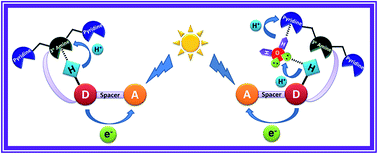
Light induced multisite electron proton transfer in two different phenol (simple and phenol carrying an intramolecularly hydrogen bonded base) pendants on acridinedione dye (ADD) and an NADH analogue was studied by following fluorescence quenching dynamics in an ultrafast timescale. In a simple phenol derivative (ADDOH), photo-excited acridinedione acquires an electron from phenol intramolecularly, coupled with the transfer of a proton to solvent water. But in a phenol carrying hydrogen bonded base (ADDDP), both electron and proton transfer occur completely intramolecularly. The sequence of this electron and proton transfer process was validated by discerning the pH dependency of the reaction kinetics. Since photo-excited ADDs are stronger oxidants, the sequential electron first proton transfer mechanism (ETPT) was observed in ADDOH and hence there is no change in the PCET reaction kinetics kETPT ∼ 6.57 × 109 s−1 in the entire pH range (pH 2–12). But the phenol carrying hydrogen bonded base (ADDDP) unleashes concerted electron proton transfer where the PCET reaction rate decreases upon decreasing the pH below its pKa. Noticeably, the concerted EPT process in ADDDP mimics the donor side of photosystem II and it occurs by two distinct pathways: (i) through direct intramolecular hydrogen bonding between the phenol and amine, kDEPT ∼ 12.5 × 1010 s−1 and (ii) through the bidirectional hydrogen bond extended by the water molecule trapped in between the proton donor and acceptor, which mediates the proton transfer and serves as a proton wire, kWMEPT ∼ 2.85 × 1010 s−1. These results unravel the incognito role played by water in mediating the proton transfer process when the structural elements do not favor direct hydrogen bonding between the proton donor and acceptor in a concerted PCET reaction.


 Please wait while we load your content...
Please wait while we load your content...