Nickel-catalyzed difunctionalization of allyl moieties using organoboronic acids and halides with divergent regioselectivities†
Abstract
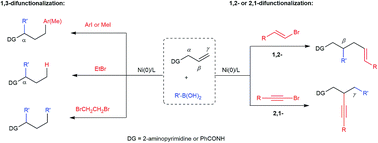
Efficient difunctionalization of alkenes allows the rapid construction of molecular complexity from simple building blocks in organic synthesis. We present herein a nickel-catalyzed dicarbofunctionalization of alkenes using readily available organoboronic acids and organic halides in a three-component fashion. In particular, an unprecedented regioselectivity of the 1,3-dicarbofunctionalization of N-allylpyrimidin-2-amine is achieved when aryl and methyl iodides are utilized. In contrast, the use of alkyl bromides with β-hydrogens results in 1,3-hydroarylation or oxidative 1,3-diarylation. Preliminary mechanistic studies suggest an isomerization involving nickel hydride in the 1,3-difunctionalization reactions. On the other hand, the use of alkenyl or alkynyl halides promotes alternative regioselectivities to deliver 1,2-alkenylcarbonation or intriguing 2,1-alkynylcarbonation products. Such 2,1-alkynylarylation is also applicable to N-allylbenzamide as a different class of substrates. Overall, this nickel-catalyzed process proves to be powerful in delivering versatile difunctionalized compounds using readily available reagents/catalysts and a simple procedure.
- This article is part of the themed collection: Most popular 2018-2019 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...