First use of a divalent lanthanide for visible-light-promoted photoredox catalysis†
Abstract
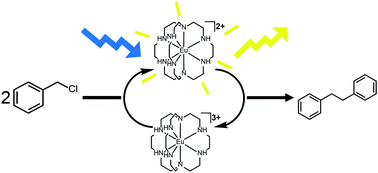
We report the first catalytic use of a divalent lanthanide in visible-light-promoted bond-forming reactions. Our new precatalyst uses europium in the +2 oxidation state and is active in the presence of blue light from light-emitting diodes. The use of low-energy visible light reduces the occurrence of potential side reactions that might be induced by higher-energy UV light. The system described here uses zinc metal as a sacrificial reductant and is tolerant to wet, protic solvents. The catalyst can be made in situ from relatively inexpensive and air-stable EuCl3·6H2O, and the ligand can be synthesized in large quantities in two steps. With 0.5% loading of precatalyst, an average of 120 turnovers was observed in six hours for reductive coupling of benzyl chloride. We expect that the results will initiate the study of visible-light-promoted photoredox catalysis using divalent europium in a variety of reactions.


 Please wait while we load your content...
Please wait while we load your content...