Asperphenamate biosynthesis reveals a novel two-module NRPS system to synthesize amino acid esters in fungi†
Abstract
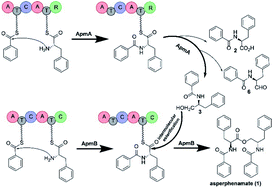
Amino acid esters are a group of structurally diverse natural products with distinct activities. Some are synthesized through an inter-molecular esterification step catalysed by nonribosomal peptide synthetase (NRPS). In bacteria, the formation of the intra-molecular ester bond is usually catalysed by a thioesterase domain of NRPS. However, the mechanism by which fungal NRPSs perform this process remains unclear. Herein, by targeted gene disruption in Penicillium brevicompactum and heterologous expression in Aspergillus nidulans, we show that two NRPSs, ApmA and ApmB, are sufficient for the synthesis of an amino acid ester, asperphenamate. Using the heterologous expression system, we identified that ApmA, with a reductase domain, rarely generates dipeptidyl alcohol. In contrast, ApmB was determined to not only catalyse inter-molecular ester bond formation but also accept the linear dipeptidyl precursor into the NRPS chain. The mechanism described here provides an approach for the synthesis of new small molecules with NRPS as the catalyst. Our study reveals for the first time a two-module NRPS system for the formation of amino acid esters in nature.


 Please wait while we load your content...
Please wait while we load your content...