Sequential double C–H functionalization of 2,5-norbornadiene in flow†
Abstract
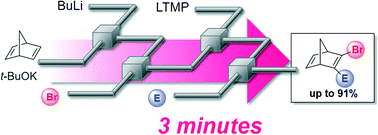
An anionic species of 2,5-norbornadiene was generated and brominated using a flow reactor. In the second step, 2-bromo-2,5-norbornadiene was lithiated with LTMP and reacted with tributyltin chloride. Finally, an integrated one-flow synthesis using various electrophiles was achieved within 3 min to prepare 2-bromo-2,5-norbornadienes bearing a functional group at the 3-position.


 Please wait while we load your content...
Please wait while we load your content...