Bubble formation in catalyst pores; curse or blessing?†
Abstract
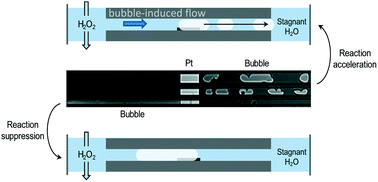
H2O2 decomposition experiments on Pt were performed in a glass microreactor, simulating arrays of catalyst pores. The formation of bubbles inside the model nanopores was observed with an optical microscope. It was found that the bubble initiation time strongly depends on the diffusion length and the H2O2 concentration. The amount of catalyst did not have a significant effect, suggesting that the reaction is diffusion limited. Results show that bubble formation can decrease the reaction rate by physically blocking the active sites, but also can accelerate the reaction by creating a forced convective flow inside the nanochannels due to bubble migration. Similar behaviour is likely to occur in a real catalyst and thus, a smart design of the catalytic support could be used to enhance reaction rates.


 Please wait while we load your content...
Please wait while we load your content...