Enhanced hydroformylation of 1-octene in n-butane expanded solvents with Co-based complexes†
Abstract
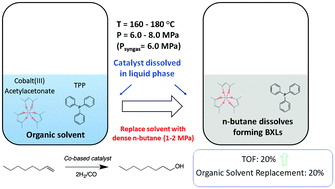
The use of n-butane expanded liquids (BXLs) as reaction media to enhance Co-catalyzed hydroformylation of 1-octene has been successfully demonstrated. Both 1-octene as well as typical hydroformylation mixtures are volumetrically expanded by n-butane at typical hydroformylation conditions (∼50% expansion at 1.8 MPa of n-butane and 180 °C). By replacing up to 20% of the traditional solvent (toluene) in the reaction mixture with compressed n-butane, the TOF for Co-catalyzed 1-octene hydroformylation with triphenylphosphine ligand was enhanced by approximately 20% in the BXL system. The higher TOF in BXLs is attributed to the improved syngas availability in the pressure-tunable BXL phase compared to the traditional liquid phase. The main impediment to TOF enhancement is catalyst precipitation beyond a certain level of n-butane dissolution in the liquid phase. Hence, catalyst complexes that show improved solubility in the BXL phase are desirable to better harness the potential benefits offered by gas-expanded liquids.


 Please wait while we load your content...
Please wait while we load your content...
