A definitive assessment of the CO oxidation pattern of a nanocomposite MnCeOx catalyst†
Abstract
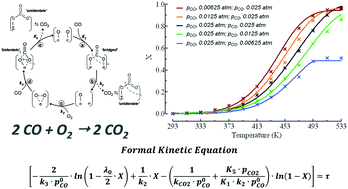
The CO oxidation pattern of a nanocomposite MnCeOx catalyst (M5C1; Mnat/Ceat, 5) in the range of 293–533 K (P, 1 atm) has been probed under a kinetic regime, varying reagent pressure (p0CO, 0.01–0.025 atm; λ0, 1), 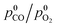 ratio (λ0, 0.25–4.0) and CO2 co-feeding (0.05–0.10 atm). Activity data indicate fractional orders on pCO (0.6 ± 0.1) and pO2 (0.4 ± 0.1), with an activation energy of 40 ± 3 kJ mol−1, and a negative kinetic effect of CO2 co-feeding due to competitive adsorption processes. Coupled with systematic evidence on the reactivity and mobility of catalyst oxygen and surface intermediates, kinetic data disclose a concerted redox mechanism of Langmuir–Hinshelwood type, which starts by abstraction of O-atoms from surface active MnIV centres (r.d.s.), and is sustained by adsorption of diatomic oxygen species on O-vacancies. The derivative and integral forms of the formal rate equation explain the empirical kinetics, predicting the activity pattern of the MnCeOx catalyst in the range of 293–533 K.
ratio (λ0, 0.25–4.0) and CO2 co-feeding (0.05–0.10 atm). Activity data indicate fractional orders on pCO (0.6 ± 0.1) and pO2 (0.4 ± 0.1), with an activation energy of 40 ± 3 kJ mol−1, and a negative kinetic effect of CO2 co-feeding due to competitive adsorption processes. Coupled with systematic evidence on the reactivity and mobility of catalyst oxygen and surface intermediates, kinetic data disclose a concerted redox mechanism of Langmuir–Hinshelwood type, which starts by abstraction of O-atoms from surface active MnIV centres (r.d.s.), and is sustained by adsorption of diatomic oxygen species on O-vacancies. The derivative and integral forms of the formal rate equation explain the empirical kinetics, predicting the activity pattern of the MnCeOx catalyst in the range of 293–533 K.


 Please wait while we load your content...
Please wait while we load your content...