Catalytic activity dependence on morphological properties of acidic ion-exchange resins for the simultaneous ETBE and TAEE liquid-phase synthesis
Abstract
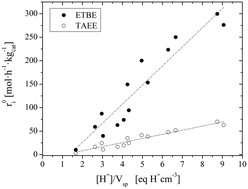
The simultaneous liquid-phase synthesis of 2-ethoxy-2-methylpropane (ETBE) and 2-ethoxy-2-methylbutane (TAEE) has been studied over fifteen commercial acidic ion-exchange resins. Kinetic experiments were carried out in a batch reactor at T = 335 K and initial molar ratios of alcohol to olefins (R°A/O) and between olefins (R°C4/C5) of 1.1 and 1, respectively. The catalytic activity, measured as intrinsic initial etherification rates, has been found to decrease in the order: Amberlyst™ 35 > Amberlyst™ 48 > Purolite® CT-275 > Amberlyst™ 15 > Purolite® CT-175 > Amberlyst™ 40 > Amberlyst™ 36 > Amberlyst™ 16 > Purolite® CT-482 > Amberlyst™ 39 > Amberlyst™ DT > Amberlyst™ 45 > Purolite® CT-124 > Purolite® MN-500 > Amberlyst™ 46. This catalytic activity rank is related to the morphological properties of the resins in both dry and swollen states. The ratio of acid capacity to specific volume of the swollen polymer has been found to be the main catalyst properties that determine their activity: the higher the ratio, the higher the activity.


 Please wait while we load your content...
Please wait while we load your content...