Simulation of nitrogen transformation in pressurized oxy-fuel combustion of pulverized coal
Abstract
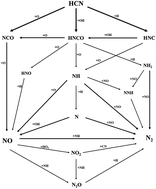
Chemical kinetic modeling was applied to simulate N transformation in the pressurized oxy-fuel combustion process of pulverized coal. Modeling accuracy was validated by experimental data at different operation pressures. The key reaction paths from fuel-N to different N products were revealed by analyzing the rate of production. NO formation was synergistically affected by six elementary reactions, in which NCO and other intermediate species were involved. The reactions among N, NH, NH2, and NO were the key paths of N2 formation. After pressurizing the combustion system, NO and N2 contents decreased and increased, respectively. High operation pressure inhibited the diffusion of NO from the internal to the external part of char. This condition prolonged the residence time of NO inside the char, triggered a typical heterogeneous reaction between gaseous NO and unburned char, and reduced the conversion from fuel-N to NO. Moreover, modeling was performed to predict NOx emission in pressurized oxy-fuel combustion as a function of various operating parameters, including temperature and excess air and recycling ratios. This study may provide guidance for reducing NOx emissions and improving combustion efficiency in oxy-fuel combustion, and it can serve as a reference for industrial applications that involve pulverized coal combustion.


 Please wait while we load your content...
Please wait while we load your content...