Three-dimensional mesoporous calcium carbonate–silica frameworks thermally activated from porous fossil bryophyte: adsorption studies for heavy metal uptake
Abstract
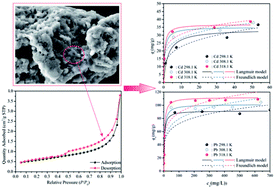
In this paper, three-dimensional mesoporous calcium carbonate–silica frameworks have been created from the straw tufa (ST) originating from porous fossil bryophyte by a thermal activation technique. A batch of adsorption kinetic and thermodynamic experiments were used to investigate the adsorption capacity of Cd(II) onto the samples. The ST after thermal activation has shown a significant ability for the uptake of heavy metals. It exhibited maximum adsorption capacities of 12.76 mg g−1, 14.09 mg g−1, 17.00 mg g−1, and 33.81 mg g−1 for Cd(II) at the activation temperature of 300, 450, 600 and 750 °C, respectively. Through competitive adsorption for Cd(II)and Pb(II), the ST thermally activated at 750 °C exhibited maximum equilibrium adsorption capacities of 24.65 mg g−1, 25.91 mg g−1, and 30.94 mg g−1 for Cd(II) uptake at 298.1 K, 308.1 K and 318.1 K, respectively, whereas it exhibited values of 91.59 mg g−1, 101.32 mg g−1, and 112.19 mg g−1 for Pb(II) removal. The adsorption capacities of Cd(II) and Pb(II) both decrease with the addition of the other heavy metal cations, indicating that the adsorption is hindered by the competitive adsorption and the adsorption active sites on the mineral surface are readily exchangeable. The adsorption of Cd(II) and Pb(II) followed the pseudo-second order kinetics model well. In addition, the Langmuir model could accurately describe the adsorption isotherms. Based on the results of characterization with TEM, XRD and XPS, the adsorption mechanisms could be primarily explained as formation of Cd(OH)2 and CdCO3 as well as Cd(HCO3)2 precipitation on the surface of ST. These characteristics of ion-exchange and the adsorptive property for ST modified allow it to be widely used in artificial wetland landfill and environmental protection.


 Please wait while we load your content...
Please wait while we load your content...