Impact of bulky phenylalkyl substituents on the air-stable n-channel transistors of birhodanine analogues†
Abstract
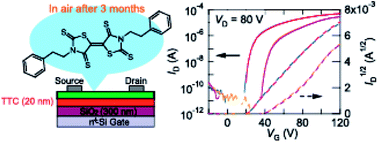
By introducing bulky 2-phenylethyl groups into sulfur-rich electron acceptors, 5,5′-bithiazolidinylidene-2,2′-dione-4,4′-dithione and 5,5′-bithiazolidinylidene-2,4,2′,4′-tetrathione, electron transport with the mobility of 0.27 cm2 V−1 s−1 with ambient and long-term stability is achieved in thin-film transistors. Bulky groups destroy the intermolecular S–S network, but the long-term transistor stability is maintained. Here, benzyl groups realize one-dimensional stacking structures, whereas 2-phenylethyl groups lead to herringbone structures.


 Please wait while we load your content...
Please wait while we load your content...