Silicon-coordinated nitrogen-doped graphene as a promising metal-free catalyst for N2O reduction by CO: a theoretical study†
Abstract
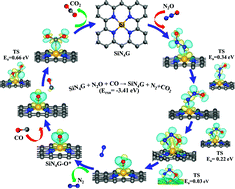
Metal-free catalysts for the transformation of N2O and CO into green products under mild conditions have long been expected. The present work proposes using silicon-coordinated nitrogen-doped graphene (SiN4G) as a catalyst for N2O reduction and CO oxidation based on periodic DFT calculations. The reaction proceeds via two steps, which are N2O reduction at the Si reaction center, producing Si–O*, which subsequently oxidizes CO to CO2. The N2O reduction occurs with an activation energy barrier of 0.34 eV, while the CO oxidation step requires an energy of 0.66 eV. The overall reaction is highly exothermic, with a reaction energy of −3.41 eV, mostly due to the N2 generation step. Compared to other metal-free catalysts, SiN4G shows the higher selectivity because it not only strongly prefers to adsorb N2O over CO, but the produced N2 and CO2 are easily desorbed, which prevents the poisoning of the active catalytic sites. These results demonstrate that SiN4G is a promising metal-free catalyst for N2O reduction and CO oxidation under mild conditions, as the reaction is both thermodynamically and kinetically favorable.


 Please wait while we load your content...
Please wait while we load your content...