Maleimide end-functionalized poly(2-oxazoline)s by the functional initiator route: synthesis and (bio)conjugation†
Abstract
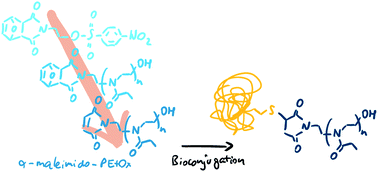
The synthesis of poly(2-ethyl-2-oxazoline)s with a maleimide group at the α chain end was carried out from new sulfonate ester initiators bearing a furan-protected maleimide group. The conditions of the polymerization were optimized for 50 °C using conventional heating (in contrast to microwave irradiation) to counteract the thermal lability of the cycloadduct introduced to protect the maleimide double bond. At this temperature, a tosylate variant was found to be unable to initiate the polymerization after several days. The controlled polymerization of 2-ethyl-2-oxazoline with a nosylate derivative was, however, successful as shown by kinetic experiments monitored by gas chromatography (GC) and size-exclusion chromatography (SEC). Poly(2-ethyl-oxazoline)s of various molar masses (4500 < Mn < 12 000 g mol−1) with narrow dispersity (Đ < 1.2) were obtained. The stability of the protected maleimide functionality during the polymerization, its deprotection, and the reactivity of the deprotected end group by coupling with a model thiol molecule were proven by 1H NMR spectroscopy and electrospray ionization mass spectrometry (ESI-MS). Finally, the conjugation of maleimide-functionalized poly(2-oxazoline) to a model protein (bovine serum albumin) was demonstrated by gel electrophoresis and MALDI-ToF mass spectrometry.


 Please wait while we load your content...
Please wait while we load your content...