Titanate nanotubes-bonded organosulfonic acid as solid acid catalyst for synthesis of butyl levulinate†
Abstract
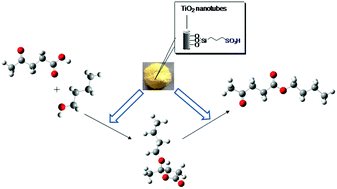
In this study, titanate nanotubes-bonded organosulfonic acid (TNTs–SO3H) was prepared and employed as an efficient heterogeneous catalyst for esterification of levulinic acid with n-butanol. Two reaction products pseudo n-butyl levulinate (pseudo-BL) and n-butyl levulinate (BL) were detected by GC-MS. The catalyst showed 86.8% conversion of levulinic acid with 99.7% selectivity towards n-butyl levulinate. TNTs–SO3H exhibited strong acidic sites and high stability even after seven cycles of usage and would be well expected to be a potential candidate for alkyl levulinate production.


 Please wait while we load your content...
Please wait while we load your content...