Redox-neutral tri-/difluoromethylation of para-quinone methides with sodium sulfinates†
Abstract
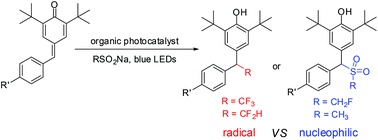
The radical tri-/difluoromethylation of para-quinone methides with readily available sodium tri-/difluoromethanesulfinate via organic photoredox catalysis is described. This reaction is external oxidant free and exhibits wide functional group compatibility, providing the desired products in useful yields. However, the reaction of para-quinone methides with CH2FSO2Na and CH3SO2Na under the optimal conditions gives the nucleophilic conjugate addition products. The results indicate that the strong inductive effect of the fluorine atom may play a critical role in the reactivity of the sodium sulfinates.


 Please wait while we load your content...
Please wait while we load your content...