Assembly of fully substituted 2,5-dihydrothiophenes via a novel sequential multicomponent reaction†
Abstract
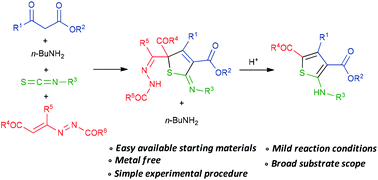
A new and efficient synthesis of fully substituted 2,5-dihydrothiophenes by a sequential one-pot four component reaction between primary amines, β-ketoesters, aryl isothiocyanates and 1,2-diaza-1,3-dienes (DDs) is reported. A careful selection of the starting materials enables the choice of up to six different variations in the architecture of the final products. Furthermore, the acidic treatment of the so-obtained 2,5-dihydrothiophenes furnishes the corresponding 5-amino thiophene-2,4-dicarboxylates.


 Please wait while we load your content...
Please wait while we load your content...