Mechanistic insight into Ni-mediated decarbonylation of unstrained ketones: the origin of decarbonylation catalytic activity†
Abstract
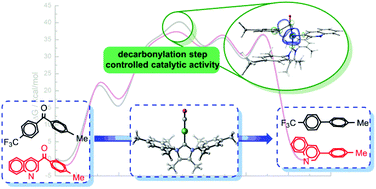
Mechanistic details of the decarbonylation of unstrained ketones by (IMesMe)Ni and (IMesMe)NiCO complexes with a N-heterocyclic carbene ligand (IMesMe) have been explored by density functional theory calculations. The calculated mechanism sheds light on the origin of realization for the decarbonylation catalytic cycle. (IMesMe)Ni mediates the first decarbonylation cycle and generates a biaryl product and (IMesMe)NiCO, which is inactive for the substrate 4-methyl-4′-trifluoromethyl benzophenone 1. However, (IMesMe)NiCO can mediate the decarbonylation of 3-quinolinyl ketone 2 and realize the catalytic cycle. The decarbonylation step is the rate-determining step controlling whether the transformation can be mediated catalytically. The stabilization energy E(2) of BDC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C(methyl phenyl) → LVNi for the decarbonylation transition state plays a dominant role in the catalytic cycle. In addition, an electron-withdrawing group on the arene can stabilize the orbital energies facilitating the initial C–C(
O)–C(methyl phenyl) → LVNi for the decarbonylation transition state plays a dominant role in the catalytic cycle. In addition, an electron-withdrawing group on the arene can stabilize the orbital energies facilitating the initial C–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) oxidative addition step. A moderate linear correlation is observed between the oxidative addition barriers and the charges transferred from (IMesMe)Ni to the benzoyl moiety.
O) oxidative addition step. A moderate linear correlation is observed between the oxidative addition barriers and the charges transferred from (IMesMe)Ni to the benzoyl moiety.


 Please wait while we load your content...
Please wait while we load your content...