Nickel-catalyzed enantioselective sequential Nazarov cyclization/decarboxylation†
Abstract
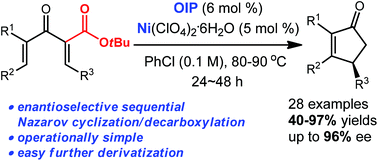
The nickel-catalyzed enantioselective sequential Nazarov cyclization/decarboxylation has been developed. The use of chiral oxazoline iminopyridine and metal perchlorate allows the enantioselective decarboxylative electrocyclization of divinyl ketones bearing an α-ester group to afford chiral cyclopentenones in good yields with excellent enantioselectivities. Various derivatizations could be easily carried out to deliver chiral polysubstituted cyclopentenes. Primary mechanistic studies demonstrated that nickel-catalyzed asymmetric Nazarov cyclization was followed by nickel-promoted decarboxylation.


 Please wait while we load your content...
Please wait while we load your content...