Synthesis of fused benzimidazoles via successive nucleophilic additions of benzimidazole derivatives to arynes under transition metal-free conditions†
Abstract
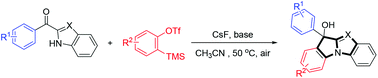
An expedient and efficient strategy was developed for the synthesis of benzimidazole derivatives from aryne precursors with CsF and K2CO3 under transition metal-free conditions. The reaction exhibited good functional group tolerance, as substrates containing alkyls, ethers, nitryls, halogens etc., could all undergo the reaction smoothly, and afforded the desired products in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...