Computational study on GaCl3-mediated reactions of donor–acceptor cyclopropanes with aromatic aldehydes: mechanism and role of GaCl3 and aldehydes†
Abstract
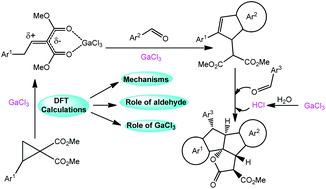
By means of density functional theory (DFT), the mechanism and role of GaCl3 and aldehydes for the GaCl3-mediated reaction of donor–acceptor (D–A) cyclopropanes and arylaldehydes have been reported in this work. DFT calculations reveal that the title reaction proceeds through three main steps: (1) the isomerization of 2-arylcyclopropane-1,1-dicarboxylates r1 to generate the ethylidenemalonate gallium complex 1a or 2-styrylmalonates 1b. (2) Subsequently, reaction between 1a (or 1b) and aromatic aldehydes would induce the formation of substituted indenes (Stage I). (3) Finally, reaction between the formed substituted indenes and aromatic aldehydes would give the polycyclic lactones (Stage II). According to our calculation, aldehydes not only participate in the reaction as a reactant, they can also promote the reaction as a co-catalyst for their proton-shuttle feature. For this reason, some excess of aldehyde is essential for implementing the process. The excess GaCl3 will hydrolyze with the water formed in Stage I, which ensures that the reaction of Stage II is carried out under acidic conditions. Furthermore, the usual [3 + 2] cycloaddition of DACs with aromatic aldehydes has also been studied. The theoretical results not only well rationalize the experimental observations but provide insights into the details of the reaction.


 Please wait while we load your content...
Please wait while we load your content...