α,β-Diaryl unsaturated ketones via palladium-catalyzed ring-opening of cyclopropenones with organoboronic acids†
Abstract
Palladium-catalyzed ring-opening acylation of cyclopropenones with organoboronic acids at room temperature has been developed. Both arylboronic acids and vinylboronic acids are viable in this acylation reaction, providing a new protocol to synthesise α,β-diaryl unsaturated ketones with good yields and excellent stereospecificity. Preliminary mechanistic studies indicated that a vinylpalladium intermediate was generated in this procedure.
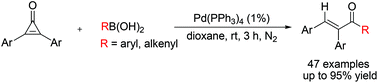


 Please wait while we load your content...
Please wait while we load your content...