Total synthesis of (±)-(1β,4β,4aβ,8aα)-4,8a-dimethyl-octahydro-naphthalene-1,4a(2H)-diol†
Abstract
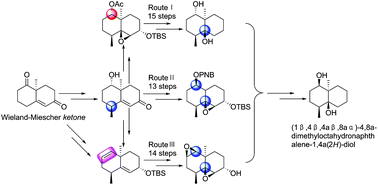
The first total synthesis of (±)-(1β,4β,4aβ,8aα)-4,8a-dimethylocta-hydronaphthalene-1,4a(2H)-diol (1), a degraded sesquiterpene isolated from a fermentation broth of Streptomyces albolongus, has been achieved via three different synthetic approaches (13–15 steps) starting from racemic Wieland–Miescher ketone (2). The configuration of hydroxyl groups at C-1 and C-4a in 1 was availably managed using the Mitsunobu reaction and stereo- and regioselective epoxidation. Moreover, the syntheses of configuration isomers 12, 17, 45 and 54 were also described in the present study.


 Please wait while we load your content...
Please wait while we load your content...