Chemoselective synthesis of aryl(pyridinyl)methanol derivatives through Ni-NIXANTPHOS catalyzed α-arylation and tandem arylation/rearrangement of pyridylmethyl ethers†
Abstract
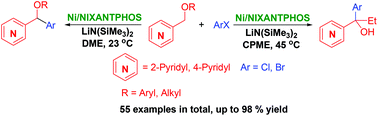
An efficient synthesis of aryl(pyridyl)-methanol derivatives using a nickel-NIXANTPHOS catalyst is described. Combinations of the Ni-NIXANTPHOS catalyst, solvent, and reaction temperature achieved chemoselective arylation and tandem arylation/rearrangement of pyridylmethyl ethers. A large variety of aryl halides were tolerated (55 examples, up to 96% yield). The scalability of the reaction is demonstrated. The order of the tandem arylation and [1,2]-Wittig rearrangement was probed by comparative studies.


 Please wait while we load your content...
Please wait while we load your content...
