Rhodium-catalyzed decarbonylative cycloadditions of 1H-indene-1,2,3-triones and alkynes via direct C–C bond activation: divergent synthesis of indenones and quinones†
Abstract
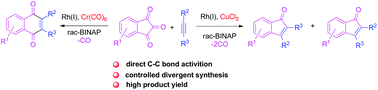
A one-step preparation method for indenone and quinone derivatives via rhodium-mediated [5 + 2 − 2] and [5 + 2 − 1] decarbonylative cycloadditions of 1H-indene-1,2,3-triones and alkynes has been achieved. A rhodium(I) complex, [Rh(COD)Cl]2, with a bisphosphine rac-BINAP ligand was the most efficient catalytic system for this decarbonylative cycloaddition transformation. The [5 + 2 − 2] and [5 + 2 − 1] transformation processes can be enhanced by respectively adding copper chloride or hexacarbonyl chromium as an additive. This reaction is suitable for a broad range of alkynes and 1H-indene-1,2,3-triones and a variety of fused indenone and quinone derivatives were obtained in medium to high yields. More importantly, this work provides a new model for the direct activation of C–C bonds in less strained ketones without auxiliary groups.


 Please wait while we load your content...
Please wait while we load your content...