A general approach for the formation of oxygen-chelated ruthenium alkylidene complexes relying on the Thorpe–Ingold effect†
Abstract
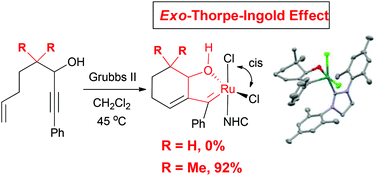
Ruthenium alkylidene complexes are prepared via enyne ring-closing metathesis relying on the exo and endo gem-dialkyl substituent effect. The structural features stabilizing oxygen-chelates are explored and confirmed by single crystal X-ray diffraction analyses. In stark contrast to the previously reported oxygen chelated Grubbs-type complexes, all chelates prepared through a ring-closing enyne metathesis regime show the disposition of the chelated oxygen and the NHC ligand in cis relationship, which force the two chloride ligands in cis-orientation. The newly synthesized oxygen-chelated ruthenium alkylidene complexes are metathesis active only at elevated temperatures.


 Please wait while we load your content...
Please wait while we load your content...
