Domino construction of a bullataketal core via double bond cleavage in activated dihydrofurans†
Abstract
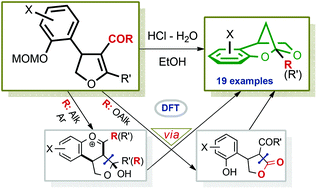
A novel approach to the tricyclic core of bullataketals A and B, natural benzannulated 2,7-dioxabicyclo[3.2.1]octanes, was developed via a domino reaction including a formal decarbonylation of 3-acyl-4-(o-hydroxyaryl)-substituted 4,5-dihydrofurans followed by spontaneous assembly of the methanobenzodioxepine skeleton. This operationally simple method provides ample opportunities for variation of substituents in the fused benzene moiety as well as at the bridgehead ketal C9 atom of the final products. The successful conversion of both 3-keto- and 3-ester-substituted dihydrofurans into functionalized methanobenzodioxepines pointed toward a substrate-controlled mechanism. This was supported experimentally and by computational studies. While 3-ester-4,5-dihydrofurans are transformed via ring opening/ring closure with further decarboxylation of the generated γ-lactone, 3-keto-4,5-dihydrofurans undergo stepwise C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond cleavage including intramolecular hemiketalization and retro-aldol reaction as the key steps.
C bond cleavage including intramolecular hemiketalization and retro-aldol reaction as the key steps.


 Please wait while we load your content...
Please wait while we load your content...