Fused donor–acceptor π-conjugated diazatruxenones: synthesis and electronic properties†
Abstract
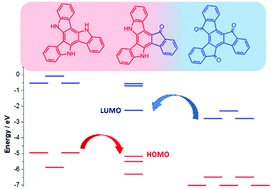
The electronic and photophysical properties of a disk-like molecule that combines two electron donor and one electron acceptor segments in a π-conjugated planar backbone, namely, 10-dihydro-15H-indeno[1,2-a]indolo[3,2-c]carbazol-15-one (diazatruxenone) have been investigated through a joint experimental and theoretical study, in order to explore its potential applications in the area of organic electronics. The influence that peripheral substitution and functionalization at the bridging nitrogens exerts on the electronic properties as well as in the supramolecular arrangement of this donor–acceptor scaffold has also been investigated. It has been found that by varying the electronic nature of the attached peripheral groups it is possible to tune its redox and optical properties. On the other hand, N-alkylation barely affects its electronic properties; however, it strongly influences how molecules interact with each other in the bulk. Interestingly, the calculated charge-transport parameters (reorganization energy and electronic coupling) confirm the potential of this disk-shaped platform to act as an ambipolar charge transporter.


 Please wait while we load your content...
Please wait while we load your content...