A highly efficient one-enzyme protocol using ω-transaminase and an amino donor enabling equilibrium displacement assisted by molecular oxygen†
Abstract
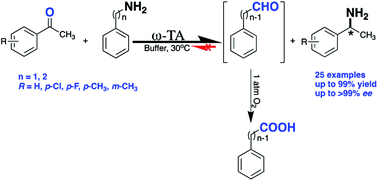
We describe a highly efficient one-enzyme procedure using ω-transaminase in the absence of expensive cofactor (NAD(P)H)-dependent enzymes used for a by-product removal process. The aromatic amino donor benzylamine, enabling its acceptance by transaminases with either (R)- or (S)-enantioselectivity, was used, allowing reactions to proceed in excellent conversions using only 3 equivalents of the amino donor. During the transamination reaction, the generated co-product aldehyde was irreversibly converted to its corresponding carboxylic acid assisted by aerobic oxidation with 1 atmosphere of oxygen, therefore the equilibrium was successfully pushed to the product side. The aerobic oxidation promoted transamination, and the corresponding method was compatible with a wide spectrum of ω-transaminases. The amino donor used here is easily and commercially obtainable, and is suitable for low-cost and scalable preparations, representing a promising pathway for clean synthesis of high value-added chiral amines.


 Please wait while we load your content...
Please wait while we load your content...