A facile hydroxylation of arylboronic acids mediated by sodium ascorbate†
Abstract
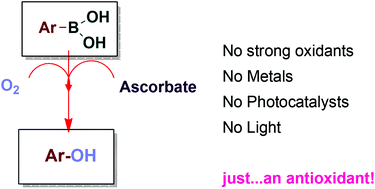
A simple, direct and facile hydroxylation of arylboronic acids has been described. The reaction is carried out under air, in an open flask, using 2 equivalents of sodium ascorbate. A variety of arylboronic acids can be transformed into the corresponding phenols in excellent to moderate isolated yields. The reaction tolerated the presence of functional groups, and molecules that are readily oxidized by H2O2 can be present in the reaction mixture. This green methodology avoids the use of photoredox conditions, transition metals, or other strong oxidants.


 Please wait while we load your content...
Please wait while we load your content...