One-pot regioselective synthesis of 2,4-disubstituted quinolines via copper(ii)-catalyzed cascade annulation†
Abstract
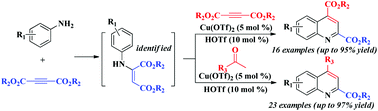
A copper(II)-catalyzed cascade annulation of simple anilines with two molecules of alkyne esters for the one-pot synthesis of 2,4-disubstituted quinolines, with exclusive regioselectivity and excellent substrate/functional group tolerance, is herein described. Moreover, the second molecule of alkyne ester in the reaction was extended to encompass (hetero)aryl- or cycloalkyl-ketone substrates, which further demonstrates the viability of the present Cu(II)-catalyzed system.


 Please wait while we load your content...
Please wait while we load your content...