Iridium/f-ampha-catalyzed asymmetric hydrogenation of aromatic α-keto esters†
Abstract
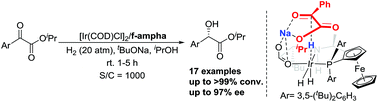
We herein report an iridium/f-ampha catalytic system for the asymmetric hydrogenation of aromatic α-keto esters. This method was demonstrated to be efficient in preparing chiral mandelate esters with up to >99% conversion and up to 97% ee. DFT calculations revealed that a quadruple Na+–O ionic interaction is formed to stabilize the transition state. The (S)-isomeric product was favored due to steric hindrance.


 Please wait while we load your content...
Please wait while we load your content...