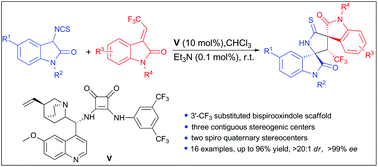
Organocatalytic Michael/cyclization cascade reactions of 3-isothiocyanato oxindoles with 3-trifluoroethylidene oxindoles: an approach for the synthesis of 3′-trifluoromethyl substituted 3,2′-pyrrolidinyl-bispirooxindoles†
Abstract
An efficient asymmetric Michael/cyclization cascade reaction of 3-isothiocyanato oxindoles with 3-trifluoroethylidene oxindoles has been revealed. Under bifunctional organocatalysis with a cinchona alkaloid derived squaramide catalyst, a series of novel 3′-trifluoromethyl substituted 3,2′-pyrrolidinyl-bispirooxindoles were conveniently constructed in a highly stereoselective manner (up to 96% yield, >20 : 1 dr and >99% ee). The reaction leads to the formation of three contiguous stereogenic centers and two spiro quaternary stereocenters.


 Please wait while we load your content...
Please wait while we load your content...